Researchers at Chalmers University of Technology, Sweden, have presented a new way to clean contaminated water using an electrochemical process. The research, Effective removal of mercury from aqueous streams via electrochemical alloy formation on platinum, published in the scientific journal Nature Communications.
“Our results have really exceeded the expectations we had when we started with the technique,” said research lead Björn Wickman, Chalmers’ Department of Physics. “Our new method makes it possible to reduce the mercury content in a liquid by more than 99 per cent. This can bring the water well within the margins for safe human consumption.”
In the last two years, Björn Wickman and Cristian Tunsu, researcher at the Department of Chemistry and Chemical Engineering at Chalmers, have studied an electrochemical process for cleaning mercury from water. The method works by extracting the heavy metal ions from water, encouraging them to form an alloy with another metal.

“Today, cleaning away the low, yet harmful, levels of mercury from large amounts of water is a major challenge. Industries need better methods to reduce the risk of mercury being released in nature,” said Wickman.
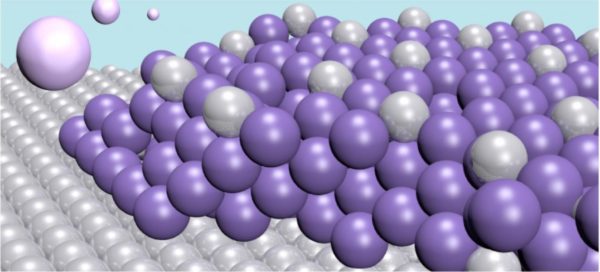
Their new method involves a metal plate—an electrode—that binds specific heavy metals to it. The electrode is made of platinum, and through an electrochemical process it draws the toxic mercury out of the water to form an alloy of the two. The alloy formed by the two metals is very stable, so there is no risk of the mercury re-entering the water.
“An alloy of this type has been made before, but with a totally different purpose in mind. This is the first time the technique with electrochemical alloying has been used for decontamination purposes,” said Cristian Tunsu.
One advantage of the technique is that the electrode has a very high capacity. Each platinum atom can bond with four mercury atoms. Furthermore, the mercury atoms do not only bond on the surface, but also penetrate deeper into the material, creating thick layers. This means the electrode has a long lifecycle. After use, it can be emptied in a controlled way. Thereby, the electrode can be recycled and the mercury disposed of safely. Another advantage is that the process is highly energy efficient.
“Another great thing with our technique is that it is very selective. Even though there may be many different types of substance in the water, it just removes the mercury. Therefore, the electrode doesn’t waste capacity by unnecessarily taking away other substances from the water,” said Wickman.
Patenting for the new method is being sought, and in order to commercialize the discovery, the new company Atium has been set up. The process has also been awarded a number of prizes and awards, both in Sweden and internationally.